Review Article
Effect of cement solidification on strength and leaching properties of Heavy Metals Contaminated Soil

Syed Taseer Abbas Jaffar*, Sajjad Yousefi Oderji, Si-an Zhang and Long-zhu Chen
Department of Civil Engineering, Shanghai Jiao Tong University, Shanghai-China, 200240, China
*Address for Correspondence: Syed Taseer Abbas Jaffar, Department of Civil Engineering, Shanghai Jiao Tong University, Shanghai-China, 200240, China, Tel: +86-18202111058; Email: [email protected]
Dates: Submitted: 01 August 2018; Approved: 10 August 2018; Published: 13 August 2018
How to cite this article: Jaffar STA, Oderji SY, Zhang S, Chen L. Effect of cement solidification on strength and leaching properties of Heavy Metals Contaminated Soil. Ann Civil Environ Eng. 2018; 2: 016-027. DOI: 10.29328/journal.acee.1001011
Copyright License: © 2018 Jaffar STA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Heavy metals; Soil pollution; Solidification; Unconfined Compressive strength
Abstract
This study investigated the effect of Portland cement on stabilization of heavy metal contaminated clayey soils that may give range of geo environemntal benefits. The absolute concentration of heavy metals: Lead (Pb), Zinc (Zn), Chromium (Cr), Cadmium (Cd) and Copper (Cu) were measured using an inductively coupled plasma atomic emission spectroscopy (ICP-AES). A series of laboratory scale experiments such as unconfined compression test (UCT), pH test and synthetic precipitation leaching procedure (SPLP) were performed to study the effects of curing time and cement content on the unconfined compressive strength (UCS) and leaching characteristics of heavy metals. According to results, excessive concentration of heavy metals are present in the topsoil of Shanghai Jiao Tong University (SJTU) among which Pb, Zn and Cd were most prominent. Other test results showed that the dry density of both C4 and C8 soil samples increases with curing time. Similarly the compressive strength (qu)of C4 and C8 samples at 21 d of curing increases by 40% (113 kPa-288 kPa) and 15% (745kPa-864 kPa) respectively, as compared to the 7 d of curing. Besides, the test results showed a prominent decrease in the leached concentration of heavy metals with increasing curing time.
Introduction
As a result of massive urbanization, soil pollution is remarkably increasing over the globe. In the recent past, it is observed that employment of systematic environmental management measures are rapidly increased to assess the soil pollution level [1-3]. Heavy metal pollution is listed as the most crucial problem among different soil pollution issues. Similarly, in China, with the fast development of urbanization, most of the pollution industries are shifted away from the residential areas [4]. Original sites where these industries have been functioning were heavily polluted by zinc, lead, and chromium because waste water discharge was not controlled properly [5]. These contaminated lands are being utilized for residential purposes in China. Irrespective of recently developed laws for reusing polluted land management in China, country still needs to develop and progress in remediation engineering practice, technical standards, and research. Both the engineers and researchers are facing this challenge of remediation of the contaminated lands in China. The rich mineral resources in China are a prime source of energy and industrial raw materials. As a result of excessive exploitation of mineral resources, a serious impact has exposed to the surrounding environment. Due to long-term water scouring and natural sedimentation, a widespread and severe soil contamination exists in urbanized areas, resulting in potential geo environmental risks [6].
Heavy metal contaminated soils can cause deterioration in the local biosphere and expose environmental threats to the eco-system and most importantly it is very dangerous for the human health [7]. It is reported that the concentrations of heavy metals Pb, Zn and Cd in the urban top soils of shanghai are 5.6, 3.0 and 2.8 times excessive than the Shanghai soil background values respectively [8]. Therefore, treatment of heavy metal polluted soils in urbanized areas is of great concern.
The commonly used technologies for the treatment of heavy metal contaminated soils include natural attenuation, isolation, and remediation [5,9]. Previous studies have investigated the use of phytoremediation and bioremediation methods to remediate the organic-contaminated industrial soils and farmland in China. Limited studies have been done on electrokinetic remediation of heavy metal contaminated soils [10]. However, remediation of contaminated soils gets limited attention; the electrokinetic remediation for lead (Pb) contaminated soils has high cost and needs comparatively longer time [11]. Heavy metal polluted soils are treated by solidification/stabilization (S/S) method over the world. The solidified soil exhibits elevated strength as compared to untreated soils, that is advantageous to improve various engineering properties such as bearing capacity, shrinking and swelling and permeability of soft, problematic and contaminated soils [12].The prevailing binders for S/S are Portland cement and cement based cementitious materials. The mechanical mixing of cement and contaminated soils and immobilizing the heavy metals by encapsulation, sorption and precipitation (e.g., metal hydroxides) are the phenomenon that are mainly involved in Solidification/stabilization by cement materials. As a result, diffusive characteristics of heavy metals will be reduced and consequently satisfy environmental standards [13,14]. Investigated the early strength characteristics of Cu-contaminated slurry solidified by Portland cement [15]. Although, numerous binders along with their mixtures have been used for the S/S treatment but cement is considered as the most desirable material among all the available materials being used for the remediation of contaminated soils [16]. However [17], indicated that the strength of the cement-solidified contaminated soils decreased with increasing Pb concentration. Found that chlorides of Mn, Co, Ni, Cu, Zn and other heavy metals could react with the silicates and aluminates in cement [18], making compounds that prevent its strength development. The acidic groundwater promotes the leaching of heavy metals like Lead (Pb), Zinc (Zn), Cadmium (Cd) and Manganese (Mn) from solidified/stabilized contaminated soil [19]. The synthetic precipitation leaching procedure (SPLP) test is widely used in many countries to investigate the leaching characteristics of pollutants in least favorable conditions in order to estimate the safety of solidified contaminated soils. In this study, a series of laboratory tests were performed on heavy metal contaminated soils solidified by cement, including unconfined compressive strength (UCS) test and synthetic precipitation leaching procedure (SPLP) test. The impacts of curing time and cement content on the moisture content, dry density, qu, concentration of leached heavy metals Cu, Cr, Pb, Zn and Cd are discussed. An empirical equation is proposed to develop a relationship between qu,t and leached concentration of heavy metals for the solidified soils.
Material and Methodology
Study area and sampling
An experimental study was carried out to evaluate heavy metal contamination and solidify heavy metal contaminated soil sampled from ten different points in Minhang campus of Shanghai Jiao Tong University (SJTU), Shanghai. Sampling depth was maintained 0-50 cm below the ground surface. From each sampling point, samples were collected by plastic shovel and preserved in a polythene bag. After air drying, samples were sieved through a 1 mm sieve and preserved in air-tight polythene bags.
Materials
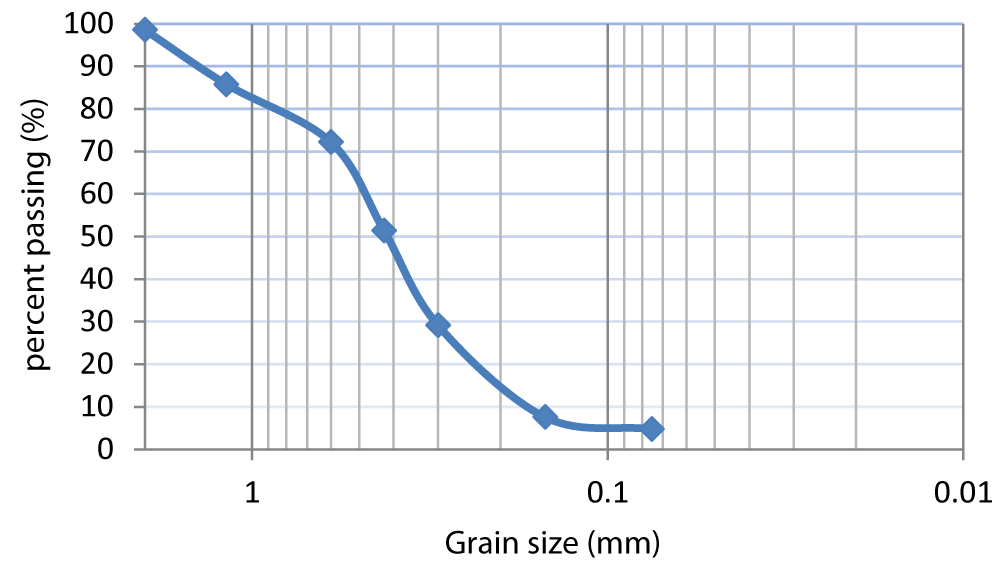
The main physical properties of soil and chemical properties of both the soil and cement sample are shown in Tables 1,2, respectively. The Atterberg limits were determined as per ASTM D4318 (ASTM 2010).Sampled soil has specific gravity 2.65 and its gradation curve is shown in Figure 1. The liquid and plastic limits are approximately 42% and 23%, respectively. Soil classification was performed according to unified soil classification system (USCS) and this soil is classified as clay with low plasticity (CL). The soil pH was determined as per ASTM D4972 (ASTM 2001). Electrical conductivity of soil was determined using Hanna conductivity meter (model HI87314). Cement used in the tests is Portland cement produced in Shanghai.
| Table 1: Major Physical Properties of the Soil (S4). | |
| Property | Value |
| Natural water content, wn (%) | 38.30 |
| Specific gravity,Gs | 2.65 |
| Plastic limit,wP(%) | 23.5 |
| Liquid limit,wL (%) | 42 |
| Soil pH | 7.96 |
| Electrical Conductivity, EC(µS/cm) | 36 |
| Table 2: Chemical Composition of soil and OPC. | ||
| Oxide | Chemical composition (%) | |
| soil | OPC | |
| Silicon dioxide (SiO2) | 57.02 | 27.40 |
| Calcium oxide (CaO) | 3.63 | 44.70 |
| Aluminum oxide (Al2O3) | 16.42 | 13.1 |
| Ferric oxide (Fe2O3) | 6.79 | 3.34 |
| Magnesium oxide (MgO) | 3.68 | 1.19 |
| Sodium oxide (Na2O) | 0.81 | 0.34 |
| Potassium oxide (K2O) | 3.59 | 1.14 |
| Sulfur trioxide (SO3) | 0.05 | 3.96 |
| Loss on ignition (LOI) | 6.43 | 0.76 |
Sample preparation
Digestion of soil samples: By using a compound solution of concentrated acids: 10ml Hydrochloric Acid (HCL), 10ml Nitric Acid (HNO3), 5ml Hydrofluoric Acid (HF) and 5ml Perchloric Acid (HClO4) digestion of oven dried soil samples (0.5g) was done in a microwave accelerated reaction system for digestion (Mars-5, CEM Company, USA) using a mixture solution of the. After digestion, cooled solution was distilled and filtered. Dilution of solution was completed with 10-15ml of distilled water. For the quality control, same procedure was adopted to prepare a standard solution without soil. Approximately 50ml of filtrate was made with distilled water and an analysis was performed for Cd. Cr, Cu, Pb and Zn using Thermo Fisher Inductively coupled plasma atomic emission spectroscopy (ICP-AES) model ICAP7600. The results showed that the soil sample-4 (S4) that was collected in the vicinity of School of Material Science and Engineering, has an excess of heavy metals Cd, Cr, Cu, Pb and Zn. S4 had higher concentration of heavy metals than Shanghai soil background values so only S4 was selected for the solidification purpose, while all other soil samples were discarded for further testing i.e. Strength and leaching characteristics.
Sample Preparation for unconfined compression test (UCT): Before preparing soil sample for unconfined compression test (UCT), the sampled soil from the contaminated site was mixed thoroughly. The air-dried mixed soil was smashed and sieved through a 2mm sieve. Distilled water with a predetermined volume was added to the soil up to water content of approximately 23%. Samples were made in cylindrical molds with an inner diameter of 40mm and a height of 80mm. Oil was uniformly applied to the inner sides of the molds before soil filling in the mold. Each soil sample had the same mass, and the filling was done in five layers. After each stage of filling, the sample was shaken manually for 2 min to remove entrapped air bubbles. The curing time selected in this study was 7, 14 and 21d. The samples were cured at a temperature 20±3° and relative humidity of 95%. Two cement contents were selected, i.e., 4% and 8% (on the basis of dry weight of soil). Hereafter through the whole text, Figure and tables, a symbol of Ci-jd shows a sample with cement content of i% and a curing time of jd. Triplicates were prepared with a specific cement content and curing time.
Test methods
For each sample prior to the unconfined compression test (UCT), its bulk density was determined. The UCT was performed as per ASTM D2166-06. After the UCT, certain mass of soil was taken from the broken samples and its moisture content was determined, that is defined as ratio of the mass of water to the mass of solid in the soil. With the noted values of bulk density and moisture content, the dry density of the sample was calculated. The broken soil samples after the UCT test were sieved through a 0.5mm sieve. About 2g soil sample was subjected to the SPLP test (solid waste-Extraction Procedure for Leaching Toxicity-Sulphoric Acid & Nitric Acid Method, SPLP) (GB 5085.3-2007). In the SPLP test, concentrated sulfuric acid and nitric acid mixture was used as extraction solution (mass ratio of sulfuric acid to nitric acid was 2:1), which had pH value of 4.20±0.05 and the solid-to-liquid ratio was 1:20. After the SPLP test, the leachate was collected and left to stand for 1h prior to the pH measurement. The pH was tested using lightning magnetic ph meter (model phs-3e) at temperature of 13 degrees. After the pH test, the leachate was filtered through a 0.45μm membrane and its supernatant (about 10 ml) was taken. The supernatant was acidized by concentrated nitric acid until its pH value reached less than 2, i.e., pH<2, and then the concentrations of leached Zn and Pb were measured using Thermo Fisher ICP-AES Plasma Emission spectrometer (model ICAP7600). Triplicates were made and the average values of concentration were recorded. Scanning Electron Microscopy (SEM) test was performed to investigate the effect of cement solidification on microstructure of soil. The soil samples were prepared according to the optimum water content and the maximum dry unit weight. A curing time of 7 days was selected for untreated and the cement-treated heavy metals contaminated soil [20]. SEM (EM-30, COXEM and South Korea) was used for this analysis.
Evaluation of soil pollution
Quality of soil was estimated according to “Environmental quality standard for soils” reported by People’s Republic of China (GB15618-1995 1995). As per this standard, soil of urbanized and industrial areas is evaluated by Class II (pH<6.5). Accordingly, Class II (pH<6.5) is used for the evaluation of soil quality in the present study. National Chinese standards for soil quality are presented in Table 3. The standards categorize the soil in three classes. National soil background values are presented in Class I. Class II shows standards for evaluation of soil from urbanized areas, agricultural areas and farmlands, etc., where living organism and environment are under serious threat of soil quality that could be directly or indirectly harmful for the human beings. Further classification of Class II is made on the basis of soil pH: acidic, neutral, alkaline. Class III is specified for forest lands.
| Table 3: Chinese National standards for soil quality (GB15618-1995 1995) (mg kg-1). | |||||
| Heavy metal (pHà) | Class I | Class II | Class III | ||
| Background | <6.5 | 6.5~7.5 | >7.5 | >6.5 | |
| Pb≤ | 35.0 | 250.0 | 300.0 | 350.0 | 500.0 |
| Cu≤ | 35.0 | 50.0 | 100.0 | 100.0 | 400.0 |
| Cr≤ | 90.0 | 150.0 | 200.0 | 250.0 | 300.0 |
| Ni≤ | 40.0 | 40.0 | 50.0 | 60.0 | 200.0 |
| Cd≤ | 0.2 | 0.3 | 0.3 | 0.6 | 1.0 |
| Zn≤ | 100.0 | 200.0 | 250.0 | 300.0 | 500.0 |
Results and Discussion
Heavy metal accumulation in soil
Table 4 presents the quantified analysis of heavy metals in the topsoil of SJTU (Minhang campus). A comparison of Shanghai soil background values [21,22] the National soil background values [22] to that of heavy metal contents in studied area is also provided in Table 4. The average values of heavy metal contents in the soil were noted as 0.21, 82, 55, 28,123mg kg-1 for Cd, Cr, Cu, Pb and Zn, respectively. When these values were compared to Shanghai soil background values, the contents of heavy metal from soil of SJTU were 1.61, 0.43, 2.3, 1.3 and 1.6 times higher for heavy metals mentioned in preceding lines. Furthermore, in comparison to National soil background values the amount of all the metals in studied area was found lower than “Environmental quality standard for soils” class II (Table 3).
| Table 4: Heavy metal contents (mg kg-1) in the topsoil of SJTU, Shanghai, and National soil background values (n=10) | ||||||
| Location | Pb | Cu | Cr | Cd | Zn | |
| SJTU (n=10) | Max | 47 | 378 | 162 | 0.316 | 232 |
| Min | 20 | 26 | 61 | 0.125 | 89 | |
| Median | 24 | 33 | 72 | 0.20 | 109 | |
| Average | 28 | 55 | 82 | 0.21 | 123 | |
| C.V.(%) | 29 | 150 | 36 | 29 | 34 | |
| Shanghai soil background values | 21.3 | 23.5 | 64.6 | 0.134 | 75.8 | |
| National soil background values | 35 | 35 | 90 | 0.2 | 100 | |
| Excessive multiples* | 1.3 | 2.3 | 0.43 | 1.61 | 1.64 | |
| *Multiples of Shanghai soil background values. | ||||||
Effect of curing time on moisture content
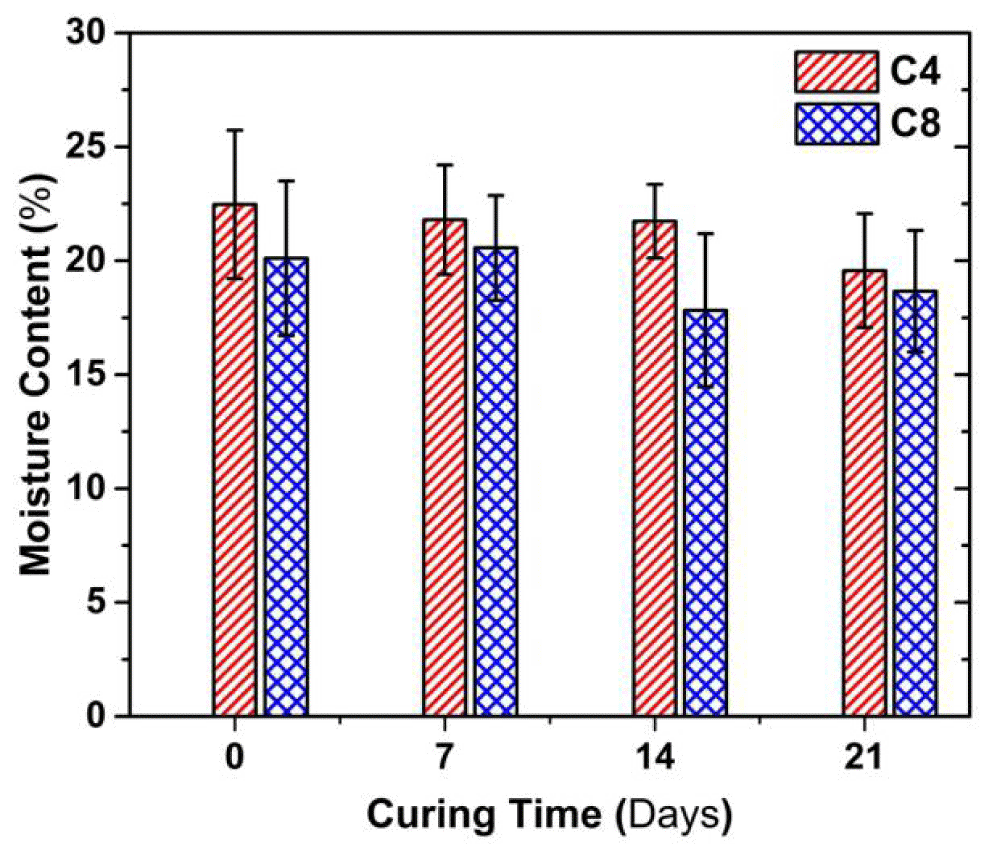
Figure 2 presents the variation in the moisture content with curing time. The moisture content of both C4 and C8 samples decreased as the curing time increased. At a given curing time, the moisture content of C8 sample is about 1.0-1.4% lower than that of C4 sample. For instance, the moisture content of C8 sample is about 1% lower than C4 sample; the difference in the moisture content is about 1.4% at 14 d of curing. When curing time increases from 0 to 21d, the moisture content of the C4 sample decreases by 13%, and that of C8 does by around 9%.
Effect of curing time on dry density
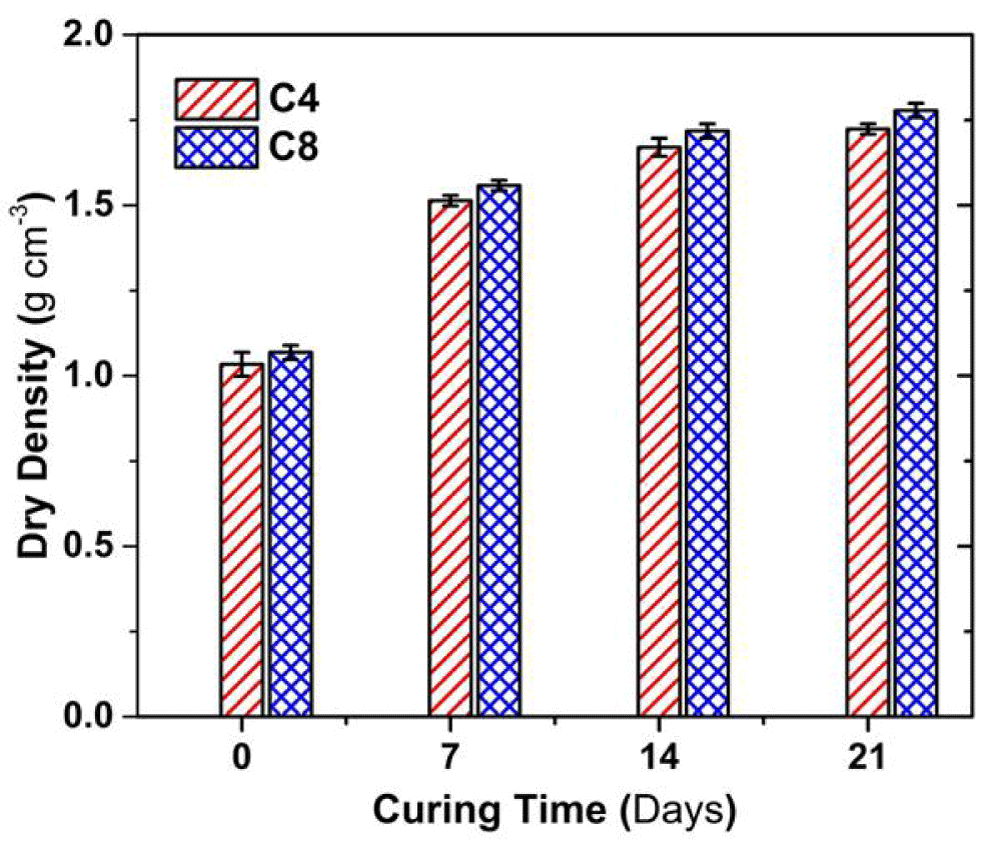
Figure 3 shows the variation in the dry density with the curing time. The dry density of both C4 and C8 soil samples increases with curing time; the increment of the dry density is within 1.2- 1.6 g cm3. Mostly, the dry density of the C8 sample was approximately 3% higher than the C4 sample at a given curing time.
Stress-strain correlation
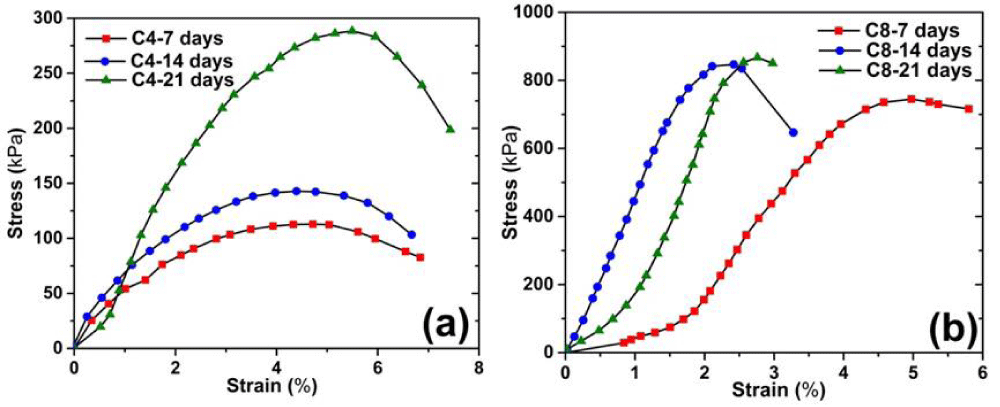
Figure 4 describes the stress-strain curves resulted from the UCT. It can be seen that the change in the stress-strain relation with respect to curing time and cement content is consistent with research literature [23]. The samples with longer curing time or higher cement content exhibit higher stress resistance to breakage, yet possess lower stress resistance once it reaches the peak, illustrating a strain softening behavior. The stress strain curves of C4 and C8 samples can be categorized into three stages: elastic deformation stage (when the stress and the strain basically show a linear relation); plastic yield stage (when the soil experiences nonlinear deformation); and the failure stage (where soil samples fails with permanent deformation). As the curing time increases, the peak stress (i.e. qu) value for both C4 and C8 samples increases as well. For instance, the peak stress of C4 sample at 14d of curing is significantly higher than 7d of curing; the peak stress of C8 sample at 21d of curing is noticeably higher than 14d of curing. The peak stress value of the C8 sample is often higher than that of the C4 sample, regardless of the curing time [24].
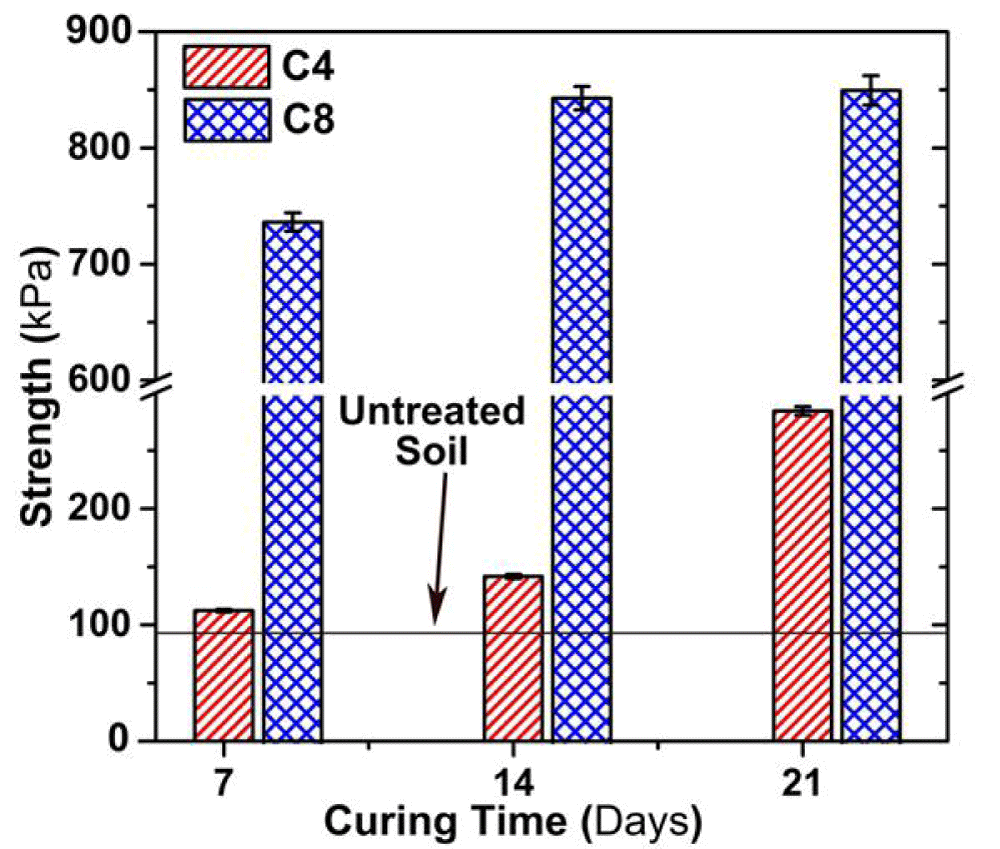
Effect of curing time on UCS
Figure 5 illustrates the variation in qu with the curing time. The qu of cement solidified soil is much greater than that of untreated contaminated soil throughout the 21d of curing. Moreover, the qu of the C8 sample is approximately 5 times higher than that of the C4 sample. The qu of C4 and C8 samples at 21d of curing increases by 40% and 15%, respectively, as compared to the 7d of curing. The qu of both C4 and C8 samples is remarkably greater than that of the untreated soil, showing a higher bonding strength of the soils admixed with cement additives.
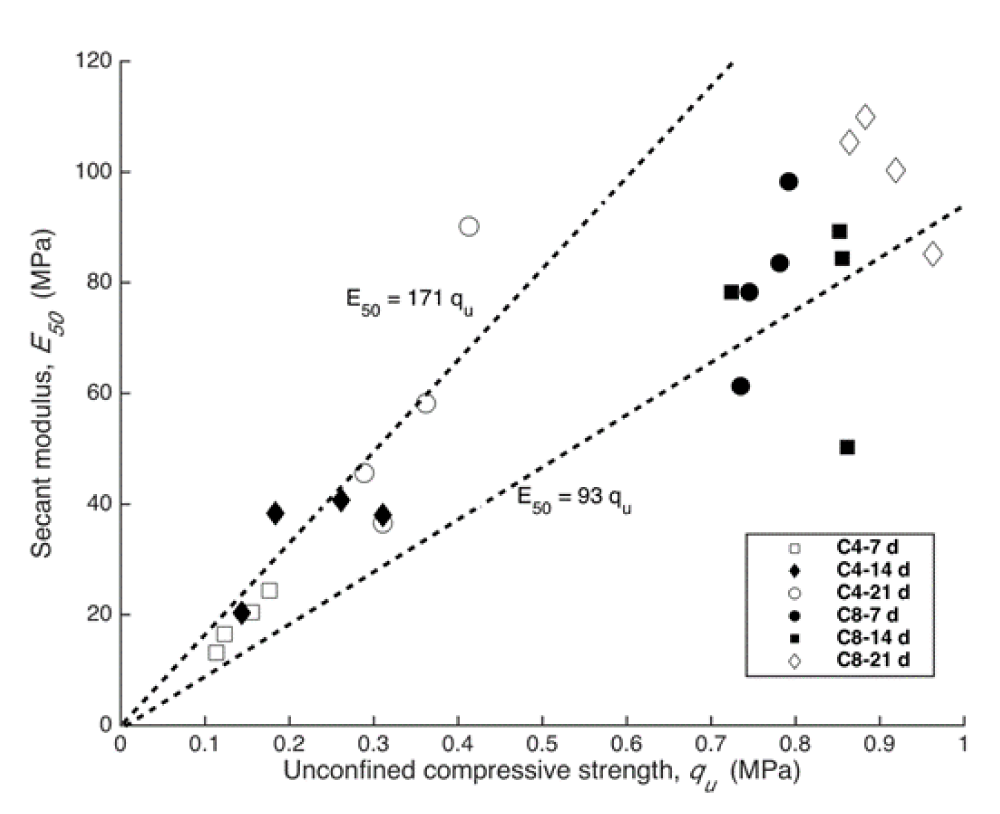
Correlation between strength and secant modulus
The secant modulus (E50) is defined as the ratio of compressive stress to its corresponding strain ε, and is expressed by
Where is stress when the strain is equal to half of the strain at failure ( ). It has been proved that qu demonstrate linear relation with E50 for cement solidified soil [24] and the relation can be written as follows
Where η is a dimension less constant.
Figure 6 describes the relation between E50 and qu for the C4 and C8 samples at curing times of 7, 14 and 21d. The η value obtained in this study ranges from 93 to 171, which is very close to the result obtained by [23,24] for cement solidified Zn-contaminated soil.
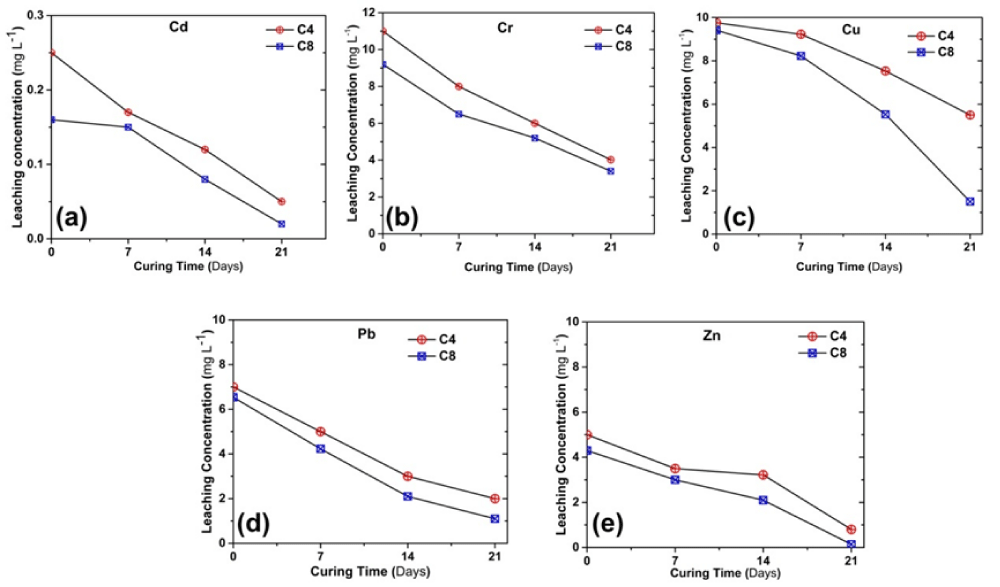
Change of heavy metals concentrations in leachate with curing time
Figure 7 shows the variation in the leached heavy metal concentration with the curing time, obtained from the SPLP tests. The heavy metal concentrations decrease with increasing curing time. It was found that the leached concentrations of all the heavy metals for the C8 samples were lower than that of the C4 samples. After 21d of curing, the Pb and Zn concentrations of C8 and C4 samples meet the environmental regulatory limits of class-II surface water (GB3838-2002). A regress analysis is conducted for identifying the correlation between qu and leached concentrations of heavy metals using MATLAB software. The derived equation is shown below.
Figure 7: SPLP test results showing the variation in the leached concentration of heavy metals (a) Cd, (b) Cr, (c) Cu, (d) Pb and (e) Zn.
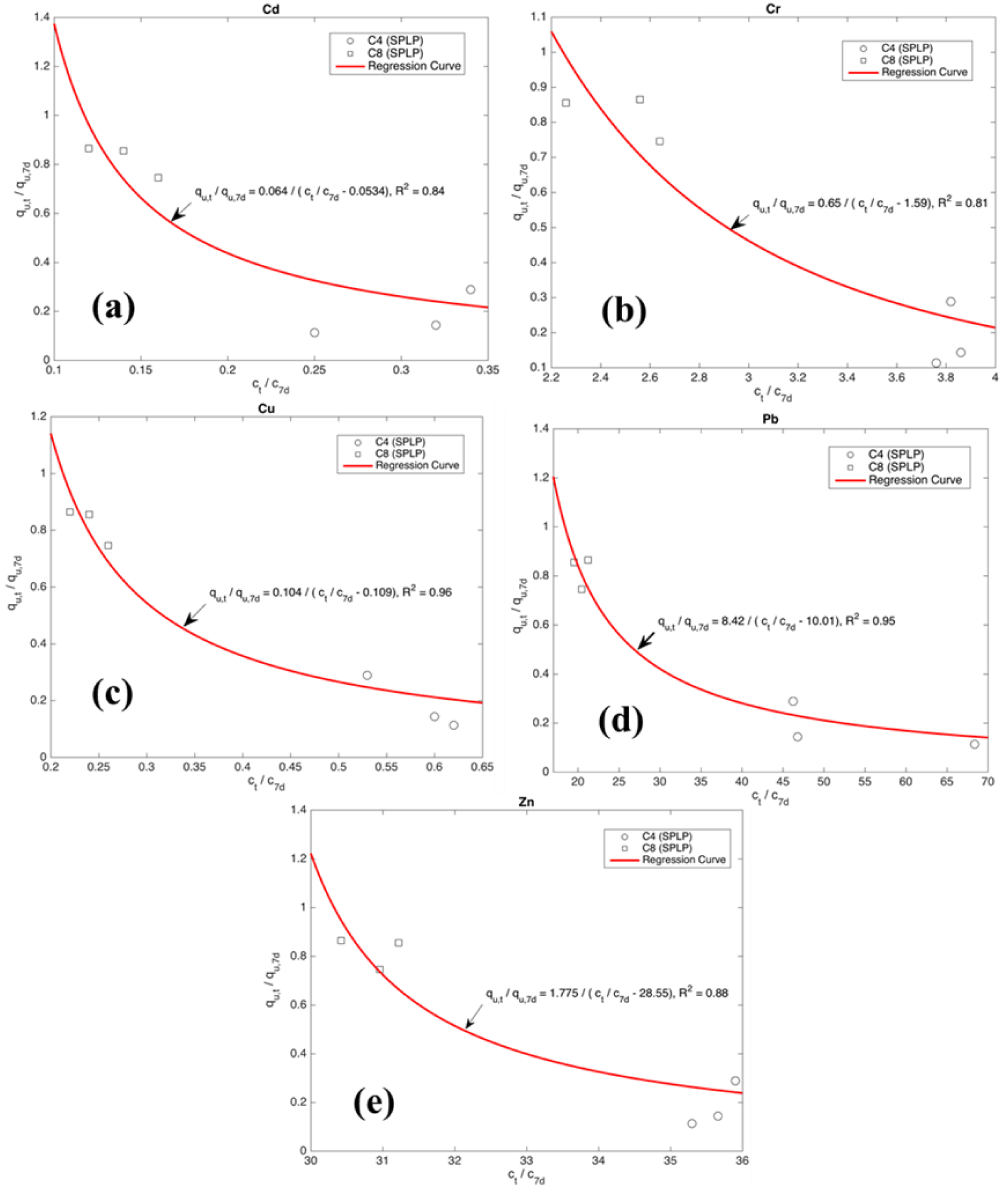
where X, Y are variables, qu,7d denotes the qu at 7d of curing; Ct denotes the leached heavy metal concentration at curing time of t (days); C7 denotes the leached metal concentration at 7 d of curing. More data is warranted to validate the general applicability of the proposed Eq. 3. Figure 8 shows the relationship between qu and leached metal concentration.
Figure 8: Relationship between qu and leached metal concentration. (a) Cd,(b) Cr, (c) Cu, (d) Pb and (e) Zn.
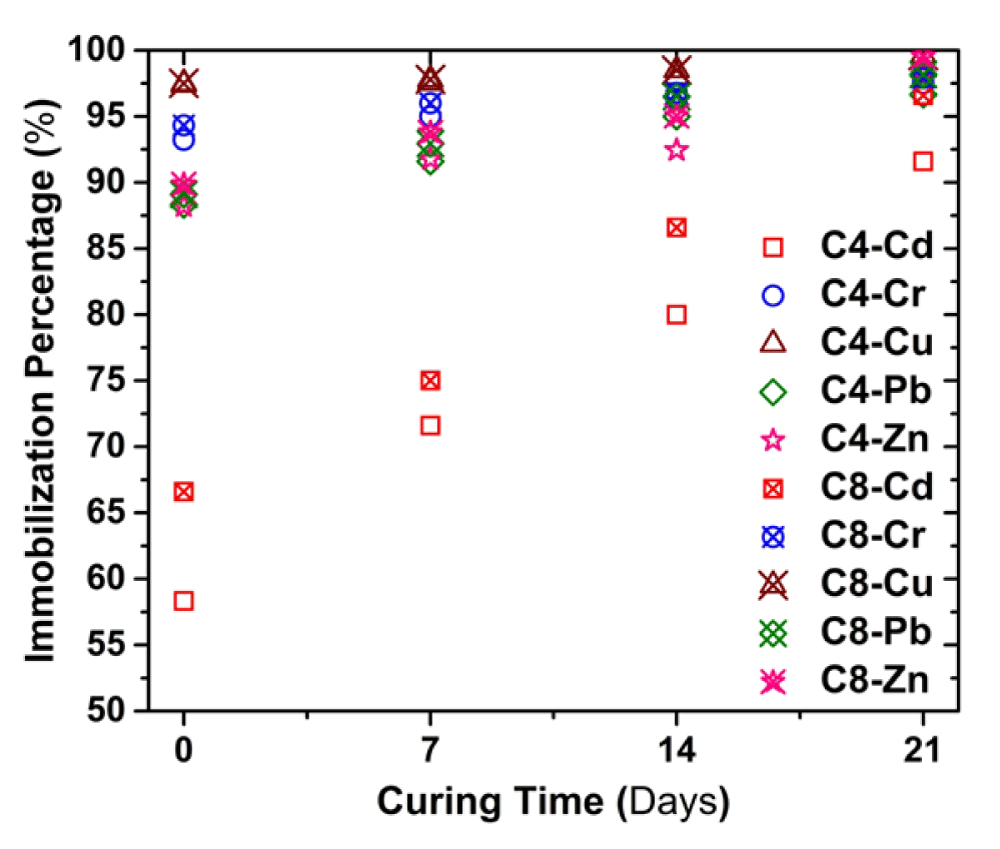
Change of Immobilization percentage with curing time
In this study, the parameter immobilization percentage (IP) was established to evaluate the effectiveness of the cement-based S/S, and it is defined by the following Eq.
Where C0 refers to the concentration of untreated contaminated soil; C1 refers to the concentration of solidified contaminated soil at a given curing time. Figure 9 illustrate the variation in IP for heavy metal with the curing time for SPLP test. It can be seen that IP of heavy metals increases with increasing curing time. At 7d of curing, the IPs of all the metals exceed 90% except Cd.
Microstructure analysis
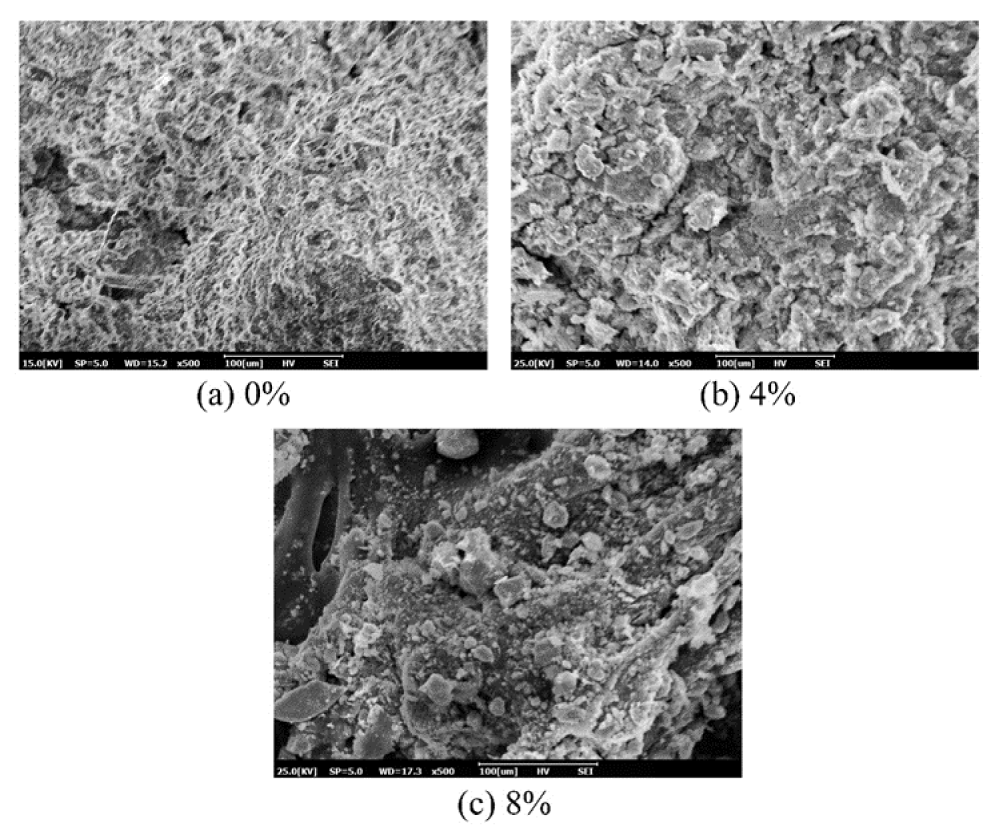
Figure 10 shows the microstructure of untreated contaminated soil and treated contaminated soil (solidified with 4 and 8% cement) for a curing time of 7 days. It can be seen from Figure 10a that soil grains have rough surface texture with semi angular shape and large voids. Such soil structures are attributed to the adsorption of the heavy metals by the soil and the change in the ions of the soil particles. Figure 10b,10c show that voids between the soil particles are filled up by the cement, which is attributed to the presence of C-S-H and ettringite that is in agreement with the previous researches [25]. Moreover, it is clear from Figure 10b,10c that the morphology of the C-S-H and ettringite became well developed as the concentration of the cement was increased from 4% to 8% in contaminated soil.
Discussion
The results reveal that the strength and leachability of the contaminated soils are significantly affected by the curing time and cement content. The observations are attributed to the cement hydration and pozzolanic reactions occurred in the cement solidified soils [26]. As the curing time increases, the two proceeding reactions consume pore water in the soils, generating calcium silicate hydrate (CSH), ettringite (AFt) and other hydration products. Resultantly, pores in the solidified soil are occupied by hydration products, promoting a reduction in moisture content and an increase in dry density with respect to curing time. The hydration and pozzolanic reactions become more intense with the increased cement content, making the moisture content lower and dry density becomes higher for of the C8 sample than that of C4 sample. The CSH, AFt and other hydration products formed in the soils can elevate their compressive strength [26]. Generally, the quantity of these hydration products increases with increasing cement content. As a result, the unconfined compressive strength of the C8 sample is higher than that of the C4 sample. Moreover, difference between rate of increment among C4 and C8 samples on the same curing times can be attributed towards the strength reducing behavior of Pb, which usually hinders the strength gaining of soil samples [27]. The immobilizations of heavy metals with cement or cement-based materials are attributed to the surface sorption of CSH, precipitations of Pb and Zn hydroxides and entry of these hydroxides into the CSH crystal lattices by substitution [28]. The amount of hydration products in the samples increases with increasing curing time; therefore, the immobilization degrees of heavy metal increase while the leached concentrations of heavy metals decreases with increasing curing time. The higher immobilization percentage of the C8 sample relative to C4 sample is attributed to the more intense hydration and pozzolanic reactions in the former case.
Conclusion
A laboratory experimental study was carried out to estimate the effects cement solidification on heavy metal contaminated soil and that was proved to be an ideal choice from several aspects. Mainly, effects of cement stabilization on strength and leaching properties of heavy metal contaminated soil were aimed. The following conclusions are drawn based on this study:
1 The unconfined compressive strength of the cement solidified soil was much higher than that of the untreated soil. The unconfined compressive strength of the C8 sample was often higher than that of the C4 sample.
2 A linear correlation between the unconfined compressive strength (qu) and secant modulus (E50) was proposed and it was expressed by E50=(93-171)qu, which is consistent with previous studies
3 The immobilization percentage of heavy metals in and SPLP tests increased as curing time increased. By the end of the 21d of curing, the leached concentrations of heavy metals of the cement solidified soils meet the China environmental regulatory limits for class-II surface water.
4 A simplified empirical equation is suggested to predict the strength of the cement solidified contaminated soil.
References
- García-Carmona M, Romero-Freire A, Aragón MS, Garzón FJM, Peinado FJM. Evaluation of remediation techniques in soils affected by residual contamination with heavy metals and arsenic. J Environ Manage. 2017; 191: 228-236. Ref.: https://tinyurl.com/yb3274pn
- Islam S, Ahmed K, Habibullah AM, Masunaga S. Potential ecological risk of hazardous elements in different land-use urban soils of Bangladesh. Sci Total Environ. 2015; 512-513: 94-102. Ref.: https://tinyurl.com/yb86pyvp
- Tang X, Li Q, Wu M, Lin L, Scholz M. Review of remediation practices regarding cadmium-enriched farmland soil with particular reference to China. J Environ Manage. 2016; 181: 646-662. Ref.: https://tinyurl.com/ydyhq42c
- Xie J, Li F. Overview of the current situation on brownfield remediation and redevelopment in China. 2010. Ref.: https://tinyurl.com/y8fjepbf
- Evanko CR, Dzombak DA. Remediation of metals-contaminated soils and groundwater: Ground-water remediation technologies analysis center Pittsburg. USA. 1997. Ref.: https://tinyurl.com/y7e8bvx4
- Zhong Sq. Soil contamination and remediation in the diggings area. Resource Development & Market. 2007; 6: 532-534.
- Jaffar STA, Luo F, Ye R, Younas H, Hu Xf, et al. The Extent of Heavy Metal Pollution and Their Potential Health Risk in Topsoils of the Massively Urbanized District of Shanghai. Arch Environ Contam Toxicol. 2017; 73: 362-376. Ref.: https://tinyurl.com/y7jys46a
- Jaffar STA, Chen Lz, Younas H, Ahmad N. Heavy metals pollution assessment in correlation with magnetic susceptibility in topsoils of Shanghai. Environmental Earth Sciences. 2017; 76: 277. Ref.: https://tinyurl.com/y93lhf55
- Khalid S, Shahid M, Niazi NK, Murtaza B, Bibi I, et al. A comparison of technologies for remediation of heavy metal contaminated soils. Journal of Geochemical Exploration. 2017; 182: 247-268. Ref.: https://tinyurl.com/ybhj98ya
- Zhu W, Li L, Lin C. Biochemical effects on permeability of solidified sludge. Yantu Lixue(Rock and Soil Mechanics). 2006; 27: 933-938. Ref.: https://tinyurl.com/y8yeukv3
- Liu YG, Li X, Zeng GM, Huang BR, Zhang HZ. Electrokinetics removal of lead from lead-contaminated red soils. Transactions of the Nonferrous Metals Society of China. 2003; 13: 1475-1478.
- Zhang T, Cai G, Liu S. Assessment of mechanical properties in recycled lignin-stabilized silty soil as base fill material. Journal of Cleaner Production, 2018; 172: 1788-1799. Ref.: https://tinyurl.com/ya4lexyp
- Riley BJ, Vienna JD, Strachan DM, McCloy JS, Jerden JL. Materials and processes for the effective capture and immobilization of radioiodine: a review. Journal of Nuclear Materials. 2016; 470: 307-326. Ref.: https://tinyurl.com/yaszzh6a
- Terashi M. Fundamental properties of lime and cement treated soils. Report of PHRI. 1980; 19: 33-62. Ref.: https://tinyurl.com/ya4mopca
- Minocha A, Jain N, Verma C. Effect of inorganic materials on the solidification of heavy metal sludge. Cement and Concrete Research. 2003; 33: 1695-1701. Ref.: https://tinyurl.com/yaevkaxm
- Goodarzi AR, Movahedrad M. Stabilization/solidification of zinc-contaminated kaolin clay using ground granulated blast-furnace slag and different types of activators. Applied Geochemistry. 2017; 81: 155-165. Ref.: https://tinyurl.com/ybt5t64n
- Lee D. Formation of leadhillite and calcium lead silicate hydrate (C–Pb–S–H) in the solidification/stabilization of lead contaminants. Chemosphere. 2007; 66: 1727-1733. Ref.: https://tinyurl.com/y7huj2q6
- Stepanova I, Lukina L, Svatovskaya L, Sychev M. Hardening of cement pastes in presence of chlorides of 3d elements. Journal of Applied Chemistry of the USSR. 1981; 54: 885-888.
- Zhang Hq, Yang Yy, Yi Yc. Effect of sulfate erosion on strength and leaching characteristic of stabilized heavy metal contaminated red clay. Transactions of Nonferrous Metals Society of China. 2017; 27: 666-675. Ref.: https://tinyurl.com/y969c9wu
- Gupta A, Arora VK, Biswas S. Contaminated dredged soil stabilization using cement and bottom ash for use as highway subgrade fill. International Journal of Geo-Engineering. 2017; 8: 20. Ref.: https://tinyurl.com/yakk9hwv
- Hu XF, Su Y, Ye R, Li XQ, Zhang GL. Magnetic properties of the urban soils in Shanghai and their environmental implications. Catena. 2007; 70: 428-436. Ref.: https://tinyurl.com/y8lnvftc
- Wei B, Yang L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchemical Journal. 2010; 94: 99-107. Ref.: https://tinyurl.com/yaxp8wma
- Du YJ, Wei ML, Jin F, Liu ZB. Stress–strain relation and strength characteristics of cement treated zinc-contaminated clay. Engineering Geology. 2013; 167: 20-26. Ref.: https://tinyurl.com/yb3tjovm
- Yang YY, Wu HL, Du YJ. Strength and leaching characteristics of heavy metal contaminated soils solidified by cement. J Residuals Science & Technology. 2014; 11: 91-98.
- Estabragh AR, Kholoosi M, Ghaziani F, Javadi AA. Mechanical and Leaching Behavior of a Stabilized and Solidified Anthracene-Contaminated Soil. Journal of Environmental Engineering. 2018; 144: 04017098. Ref.: https://tinyurl.com/y7v5w5bg
- Yan-jun D, Ning-jun J, Le W, Ming-li W. Strength and microstructure characteristics of cement-based solidified/stabilized zinc-contaminated kaolin. Chinese Journal of Geotechnical Engineering. 2012; 11: 2114-2120. Ref.: https://tinyurl.com/y9yssm3g
- Wang YS, Dai JG, Wang L, Tsang DCW, Poon CS. Influence of lead on stabilization/solidification by ordinary Portland cement and magnesium phosphate cement. Chemosphere. 2018; 190: 90-96. Ref.: https://tinyurl.com/y9nt3tex
- Gougar MLD, Scheetz BE, Roy DM. Ettringite and C-S-H portland cement phases for waste ion immobilization: A review. Waste Management. 1996; 16: 295-303. Ref.: https://tinyurl.com/yabmmohy